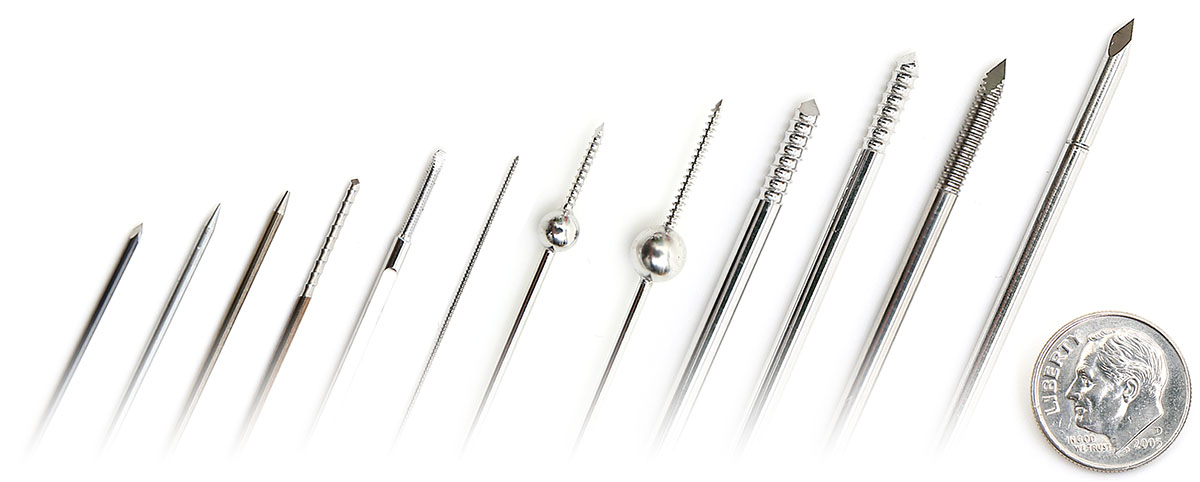
K-Wires, Steinmann Pins, & Olive Wires
Custom Wire technologies has the ability to manufacture custom orthopedic wire components for your applications. Some of these components may include Kirschner wires (k-wires), Steinmann pins, or olive wires. Stocking raw material in common sizes helps reduce the lead time when manufacturing prototypes. However, if you are looking for a specific modification beyond standard materials, sizes or an abstract design, contact our engineers about prototypes or short-runs for your specific needs. This may include threading, flats, radii, or balls.

These components are custom manufactured in strict adherence to customer specifications, primarily using certified materials such as 304V Stainless Steel, 316LVM Stainless Steel or Nitinol (Nickel Titanium). CWT produces custom wires with a variety of materials and tips. The material selection process is strictly based on customer specifications. Changes to a design can be discussed based on ease of manufacturability and cost. See below for some additional info to help define your specifications.
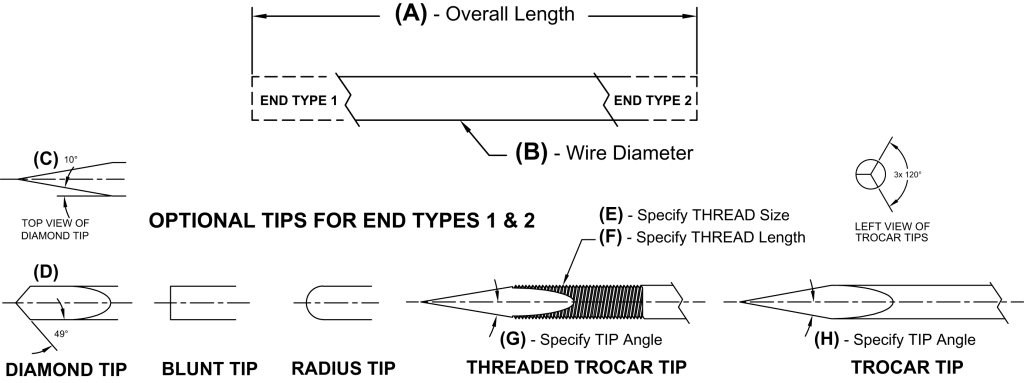
Tips:
- Trocar
- Diamond (spade)
- Radius
- Blunt
- Conical
Threads:
- Partially Threaded
- Fully Threaded
Marks:
- Laser Marking
- Ink printed bands
Finishing:
- Electropolish per ASTM B912, F86
- Laser Mark depth bands or part numbers
- Passivate per ASTM A967, F86
- PTFE Coatings
- Value Added Services